GUEST ARTICLE
Industry insights from NIZO: Precision fermentation: Making the case for ‘animal protein without the animal’

Alternative methods of producing proteins offer exciting opportunities for creating vegan alternatives to animal-based products and ingredients. Some of these even promise to create ‘animal proteins, without the animal’. But with high costs for development and production, how can you be sure you are on the right track?
René Floris, NIZO Food Research Division Manager and member of the FoodNavigator expert advisory panel, asks Herwig Bachmann, principal scientist fermentation and assistant professor at VU University, about the hurdles researchers face in taking techniques such as precision fermentation feasible.
René Floris: What are the different approaches to alternative proteins?
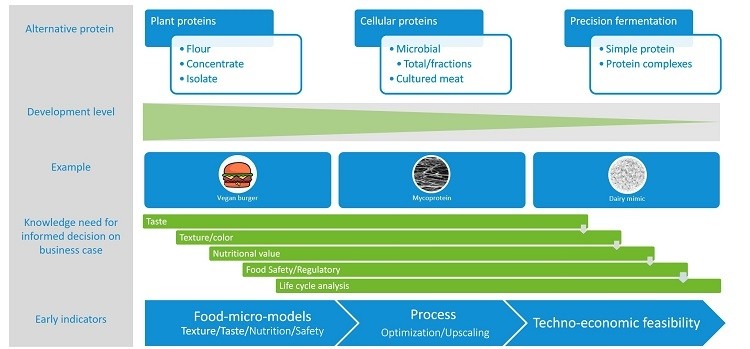
HB: Some alternative proteins aim to create ‘analogue’ products that are comparable to an animal-based product – whether meat, dairy, egg, etc – in terms of sensory characteristics, functionality and safety. Plant proteins may be the most familiar and developed of these, although cellular proteins such as mycoproteins (Quorn) from microbial biomass are also on the market.
Another approach aims to create ‘non-animal’ animal proteins: genetically the same as animal proteins, but created or grown in a lab without using actual animal products. Examples include ‘cultured meat’ (which is another type of cellular protein) and proteins created through precision fermentation.
Precision fermentation is currently the least-developed of these techniques. In this approach, typically a microorganism such as a yeast or fungus is engineered to express genes that are normally encoded by the DNA of an animal: a cow, for instance. The microorganism then expresses a specific protein, such as casein. But this casein is created without any animal products, making it vegan.
RF: What are the specific advantages of precision fermentation proteins?
HB: When you create an ‘analogue’-type protein, whether from plants, fermented biomass, etc., it has to be ‘tweaked’ and processed to get the characteristics that you want in your non-animal product. But since precision fermentation proteins are genetically the same as, say, a dairy protein, they should offer the same characteristics - if the proteins are also processed correctly in the fermentation process. They can thus be used for creating ‘animal-free’ products with the taste, texture and nutritional profile of the animal-based original.
In addition, precision fermentation aims to use fewer resources such as arable land and water, compared to animal proteins. The concept is that these proteins will be more ecologically sustainable, and eventually more economical. But we are still a long way off from that ideal.
RF: What are the challenges of developing products with precision fermentation?
HB: Currently, all techniques for creating ‘animal proteins, without the animal’ are very costly, due to intricate production processes and expensive components. In 2013, for example, a Dutch start-up introduced the world’s first cultured meat burger, based on a technology to grow muscle cells. The production cost? $325,000 for a 5-ounce (142 gram) hamburger! The producers claim that within just a few years, however, they had reduced the cost to about $11 per burger. Although at $80 per kilo, this is still quite expensive.
The production of milk casein using DNA-altering technology in a precision fermentation is similarly costly due to the need for dedicated growth media, large scale fermentors, and purification of the expressed protein. On top of that, milk is a complex product in which different proteins, minerals, sugars and fats interreact with each other: a single protein produced by precision fermentation will not act the same on its own, compared to the ‘original’ which is a complicated matrix.
With all these variables and unknowns during product development, it can be difficult to make a business case for a particular alternative protein: how do you prove you are on the right track, and get additional investment to move to the next step?
RF: Why is early testing critical for developing a precision fermentation protein?
HB: Because of the high cost of developing a precision fermentation or a cellular protein, you must be sure that your protein is good enough to use in real food products. Above all, you want to avoid developing and upscaling a very expensive protein, only to discover at the end that it either doesn’t work at all, or that the process has to be adapted. This means you should begin testing your protein as early as possible, in parallel with development, so you can adapt the process as you go along.
With some alternative proteins, such as those from plants or microbial biomass, you generally have a lot of material to work with. You develop your process in the lab, and can then scale up in order to test and optimise.
With precision fermentation, however, you initially create a very small amount of relatively expensive – and therefore very precious – material. Scaling up takes even more time and money. Yet you need enough material to prove the viability of your protein.
RF: How can the cost of development be reduced?
HB: While precision fermented proteins are scarce and costly, their original, ‘animal-sourced’ versions are readily available. You can thus use those versions to explore the potential of your precision fermented version, and try out different processes, in a kind of ‘shadow’ development.
Say you wish to develop a cheese with your precision fermentation protein. You can isolate the desired protein fraction from dairy milk, and then work out how to make it into a cheese. Which fats and carbs do you need, in which ratios, etc.? This can give you a step forward in determining how to use your -very costly – precision fermentation protein to make a real product.
RF: How can you test, with only a small amount of protein?
HB: Even if you only have a very small amount of material to work with, you can test certain characteristics: does it dissolve in water, does it gel, etc. But to evaluate technological functionalities such as taste, texture and nutritional value or food safety, you need enough material to make a basic ‘product’, to get a better understanding of the complete process. Doing this on a small scale can give you good insight into the techno-economic feasibility of your approach, and allows a better-defined lifecycle analysis of the eventual product.
Food micro-models enable you to do that with minimal material. NIZO’s validated MicroCheese model system, for example, makes and tests very small amounts of real cheese (or yoghurt) for a broad variety of consumer relevant characteristics. With MicroCheese, for example, we can test cheese made with only 1.2 ml of milk. With the right know-how, micro-food models allow rapid development and evaluation of new product categories and ingredients such as different alternative proteins.
This approach lets you evaluate the suitability of your alternative protein and the characteristics of products made with it early on - before you scale-up the protein production process. You can adapt your process if necessary, and rerun the model. And you can demonstrate to investors or management what you can expect before entering the scale up phase. So you can be sure you are on the right track, at an earlier stage of your project.
In our next article, we will look at the safety aspects of using cacao in food products.